When single cell meets spatial, researchers uncover how people with paralysis may walk again
What happens when two powerful technologies join forces to resolve the “what” and the “where” of complex biology? We share those stories in our “When single cell meets spatial” series, highlighting studies that capture the best of both technologies—and how they can be even better together.
Electrical stimulation can restore the ability to walk in paralyzed people, but why?
In 2006, Rob Summers was fresh off of a College World Series win when he was hit by a drunk driver in his own driveway (1). The doctors told the then 20-year-old he was paralyzed from the chest down and would never walk, control his bowels or bladder, or regain sexual function again.
They were wrong.
Three years later, Summers was the first person to participate in an experimental epidural electrical stimulation (EES) program to treat people with spinal cord injuries at the University of Louisville (2). A group of clinical researchers implanted a 16-electrode array on his dura and used it to electrically stimulate his lower spine—like the brain normally does to initiate movement. After nearly 30 four-hour sessions of locomotive training paired with EES, Summers could stand on his own for nearly five minutes and take steps on a treadmill with assistance while undergoing EES (3).
The researchers published their findings in The Lancet in 2008 and, at the time, were cautiously optimistic. "This is a breakthrough. It opens up a huge opportunity to improve the daily functioning of these individuals. But we have a long road ahead," said Susan Harkema, PhD, lead author of The Lancet article, in a press release posted on the same day the study was published (4).
The road got a little shorter in 2018. The clinical trial at the University of Louisville continued with three more patients, and the group reported in the New England Journal of Medicine that, after intensive stimulation and locomotive training, all four patients could stand on their own and two patients could walk with a walker or cane during EES (5). Other groups reported similar findings that year, including a group of researchers from the Swiss Federal Institute of Technology in Lausanne (6–7).
Those same researchers from the Swiss Federal Institute of Technology drove this research further down the road to the clinic in November 2022 when they reported results from their clinical trial, Stimulation Movement Overground (STIMO). They reported in Nature that, after five months of training, nine patients—who previously had no sensation in their legs—could walk during EES (8). Most remarkably, four of those patients could walk even after EES was switched off, indicating that physical therapy combined with epidural electrical stimulation could, somehow, permanently rewire neurons in the spinal cord.
Initial tests showed decreased nerve activity near the site of injury, but this didn’t surprise the researchers. “When you learn a task, that’s exactly what you see—there are less and less neurons activated as you get better at it,” said Grégoire Courtine, PhD, from the Swiss Federal Institute of Technology in Lausanne and corresponding author of the new Nature study (9).
And that’s exactly what they found. To recapitulate their study in an animal model, they examined spinal cord injuries in mice who then underwent EES. Mice with paralyzed rear legs also had dampened nerve activity at the site of injury in the spine after stimulation. But the researchers discovered that a specific subset of neurons not required for movement in wild-type mice were sufficient for movement in paralyzed mice.
A single cell atlas of the recovering spinal cord in mice using the combined power of our Chromium Single Cell Gene Expression and Visium Spatial Gene Expression kits guided them to this new potential therapeutic target for paralysis.
Defining and mapping neurons in the recovering spinal cord
The researchers first used single nucleus RNA-sequencing (snRNA-seq) to analyze spinal cord tissue from mice with and without spinal cord injuries who did or did not undergo EES. They analyzed over 80,000 nuclei from 24 mice across experimental conditions and identified all the expected major spinal cord cell types via unsupervised clustering. They zoned in on the 20,990 neuronal nuclei in the dataset and found 36 subclusters of neurons that clearly segregated into specific types of neurons, notably ventral and dorsal (Figure 1).
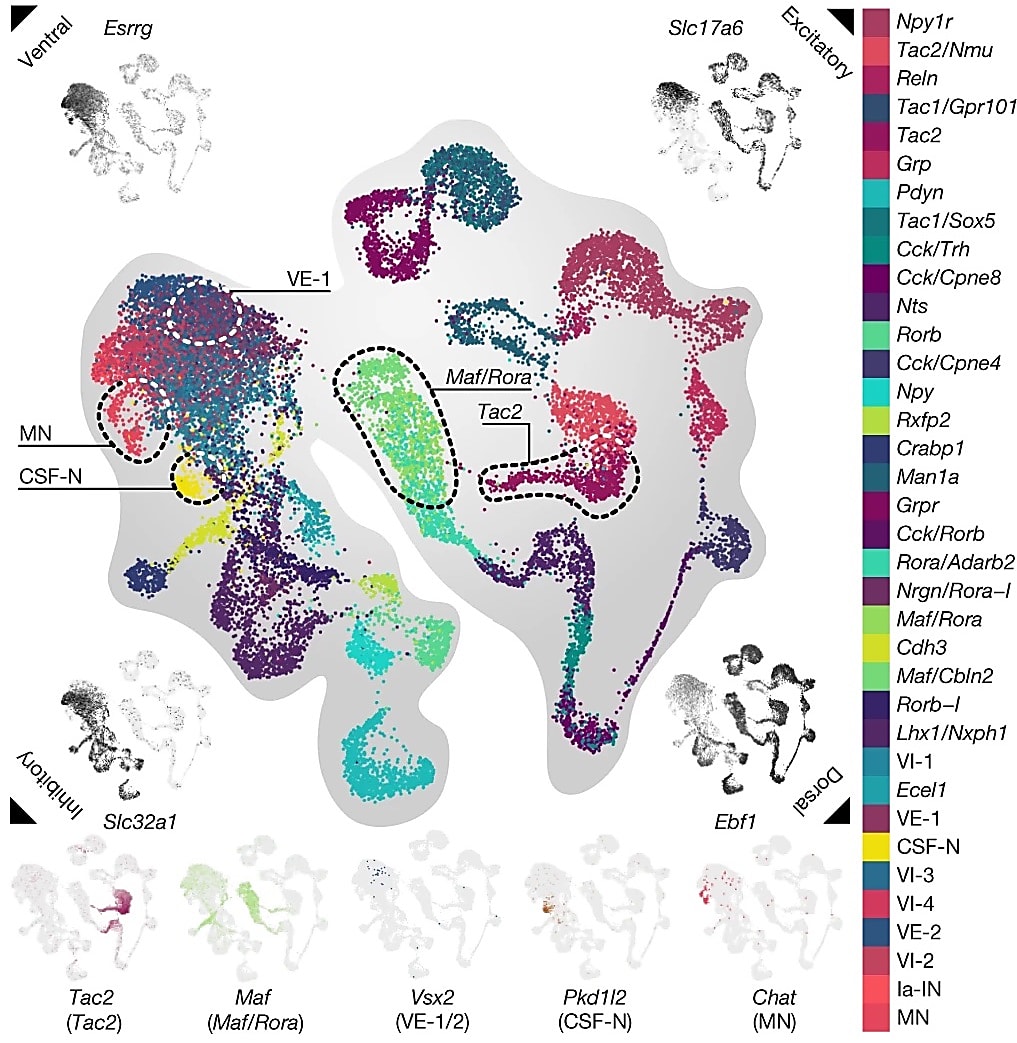
Electrical signals that dictate movement enter through dorsal neurons and exit, stimulating muscle, through ventral neurons. The distinct anatomical arrangement of these specific neurons in the spinal cord begged questions about their localization and cell–cell interactions that snRNA-seq alone could not answer. This led the researchers to use Visium Spatial Gene Expression and map the neurons in the spinal cord. They sequenced 61 sections at over 16,000 UMIs per barcode and, ultimately, mapped 22,127 barcodes within the spinal cord. Mapping all 36 neuronal populations identified by snRNA-seq, they confirmed that cells expressing ventral markers (such as Essrg) were located at the bottom of the spinal cord section and cells expressing dorsal markers (such as Ebf1) were found at the top.
“This spatially resolved single-cell atlas of the lumbar spinal cord established a molecular cartography to navigate the uncharted populations of neurons that restore walking after paralysis and thus identify recovery-organizing neurons,” wrote the authors.
The researchers developed an algorithm called Augur that, “identifies cell types that become more transcriptionally separable in response to biological perturbations.” They used this algorithm to discover the transcriptional signatures of cell populations that increased throughout the recovery trajectory of mice with spinal cord injuries treated with EES. Two populations of excitatory interneurons—cells that pass signals from sensory neurons to motor neurons—that expressed Vsx2 and Hoxa10 increased during recovery.
They created another algorithm called Magellan that used a spatial nearest neighbor framework to zone in on tissue regions that are transcriptionally altered after spatial perturbation. This method was validated through analysis of simulated datasets that applied perturbations with known transcriptional effects. Magellan identified the same neuronal populations as Augur.
Mice who recovered from spinal cord injuries lost their ability to walk when the scientists inactivated cells expressing Vsx2 and Hoxa10 using optogenetics. But inactivating these cells had no effect on mice without a spinal cord injury, suggesting this neuronal population is only essential for movement after an injury.
Jordan Squair, PhD, a postdoctoral researcher from the Swiss Federal Institute of Technology in Lausanne and a co-author of the study, told Popular Science that the mice’s spines are becoming “more efficient.” There is less neuronal activity at the site of injury, but activation of a small population of neurons in the spinal cord.
The next steps for treating paralysis
Other neuroscientists who were not involved in the study, including Michael Oh, MD, a neurosurgeon at the University of California, Irvine, are cautiously optimistic about the results of this study. “Getting people out of wheelchairs and walking independently is a miracle,” Oh told Popular Science (2).
In the future, researchers could improve spinal cord injury–related paralysis by specifically stimulating or activating neurons positive for Vsx2 and Hoxa10. Eiman Azim, PhD a neuroscientist at the Salk Institute for Biological Studies in La Jolla, California, wrote in an accompanying opinion article that electrical stimulation technology isn’t currently up to the task of such specific activation, but he’s excited to see the technology and its applications continue to improve (10).
But, Oh pointed out, this study identifies a putative cellular target for treatment, it doesn’t provide a detailed mechanism (2). Additionally, whether these findings hold up in humans is still unclear. Azim said he was optimistic because the spinal structure is similar amongst vertebrates (10).
Courtine is now ready to take his incredible single cell and spatial insights to the next level. He launched a start-up called ONWARD to commercialize the EES technology used in the study. His group is also continuing their small clinical trial STIMO, which includes the nine patients in the Nature study, and plans to recruit people with spinal cord injuries for a larger clinical trial in the US in 2024 (11).
We’re excited not only to see what this exciting research leads to, but how researchers continue to use single cell and spatial transcriptomics in mice, non-human primates, and humans to delineate the molecular underpinnings of paralysis and recovery.
Learn more about this study, and how our technology can transform your neuroscience and drug discovery research.
For more examples of the combined power of single cell and spatial transcriptomics, read the first edition of our “When single cell meets spatial” blog series.
References:
- Boyle R. With Electrical Stimulation to the Spinal Cord, Paralyzed Man Walks Again. Popular Science (2011). URL: https://www.popsci.com/science/article/2011-05/electrical-stimulation-spinal-cord-paralyzed-man-walks-again/
- Guarino B. The slow, but promising progress of electrode therapy for paralysis. Popular Science (2022). URL: https://www.popsci.com/health/paralysis-electrode-therapy-success/
- Harkema S. Effect of epidural stimulation of the lumbosacral spinal cord on voluntary movement, standing, and assisted stepping after motor complete paraplegia: a case study. The Lancet 377: P1938–1947 (2011). doi: 10.1016/S0140-6736(11)60547-3
- https://www.prnewswire.com/news-releases/paraplegic-man-stands-steps-with-assistance-and-moves-his-legs-voluntarily-122259918.html
- Angeli CA, et al. Recovery of over-ground walking after chronic motor complete spinal cord injury. N Engl J Med 379: 244–1250 (2018). doi: 10.1056/NEJMoa1803588
- Gill M L, et al. Neuromodulation of lumbosacral spinal networks enables independent stepping after complete paraplegia. Nat Med 24: 1677–1682 (2018). doi: 10.1038/s41591-018-0175-7
- Wagner FB, et al. Targeted neurotechnology restores walking in humans with spinal cord injury. Nature 563: 65–71 (2018). doi: 10.1038/s41586-018-0649-2
- Kathe C, et al. The neurons that restore walking after paralysis. Nature 611: 540–547 (2022). doi: 10.1038/s41586-022-05385-7
- Lewis D, et al. Electrical stimulation helps paralysed people walk again—and now we know why. Nature 611: 438 (2022). doi: 10.1038/d41586-022-03605-8
- Huang KW and Azim E. Neurons that promote recovery from paralysis identified. Nature (2022). URL: https://www.nature.com/articles/d41586-022-02234-5
- Heidt A. Scientists Identify Neurons Needed to Walk After Paralysis. The Scientist (2022). URL: https://www.the-scientist.com/news-opinion/scientists-identify-neurons-needed-to-walk-after-paralysis-70744
